Fuel cells have emerged as groundbreaking energy-conversion solutions with the ability to generate electricity through electrochemical reactions, without the harmful emissions associated with combustion. These cells hold immense potential in powering a wide range of technologies, from electric vehicles to portable chargers and industrial machines. However, the widespread adoption of fuel cells has been hindered by the reliance on expensive materials and precious metal catalysts in many existing designs.
Anion-exchange-membrane fuel cells (AEMFCs) offer a ray of hope in addressing the challenges faced by traditional fuel cell designs. These fuel cells are built on Earth-abundant, low-cost catalysts, making them a more affordable alternative to their predecessors. Over the years, research groups across the globe have been investing efforts in designing and testing new AEMFCs. While some of these innovations have shown promising results, a common issue encountered is the self-oxidation of non-precious metals used as catalysts, leading to irreversible cell failure.
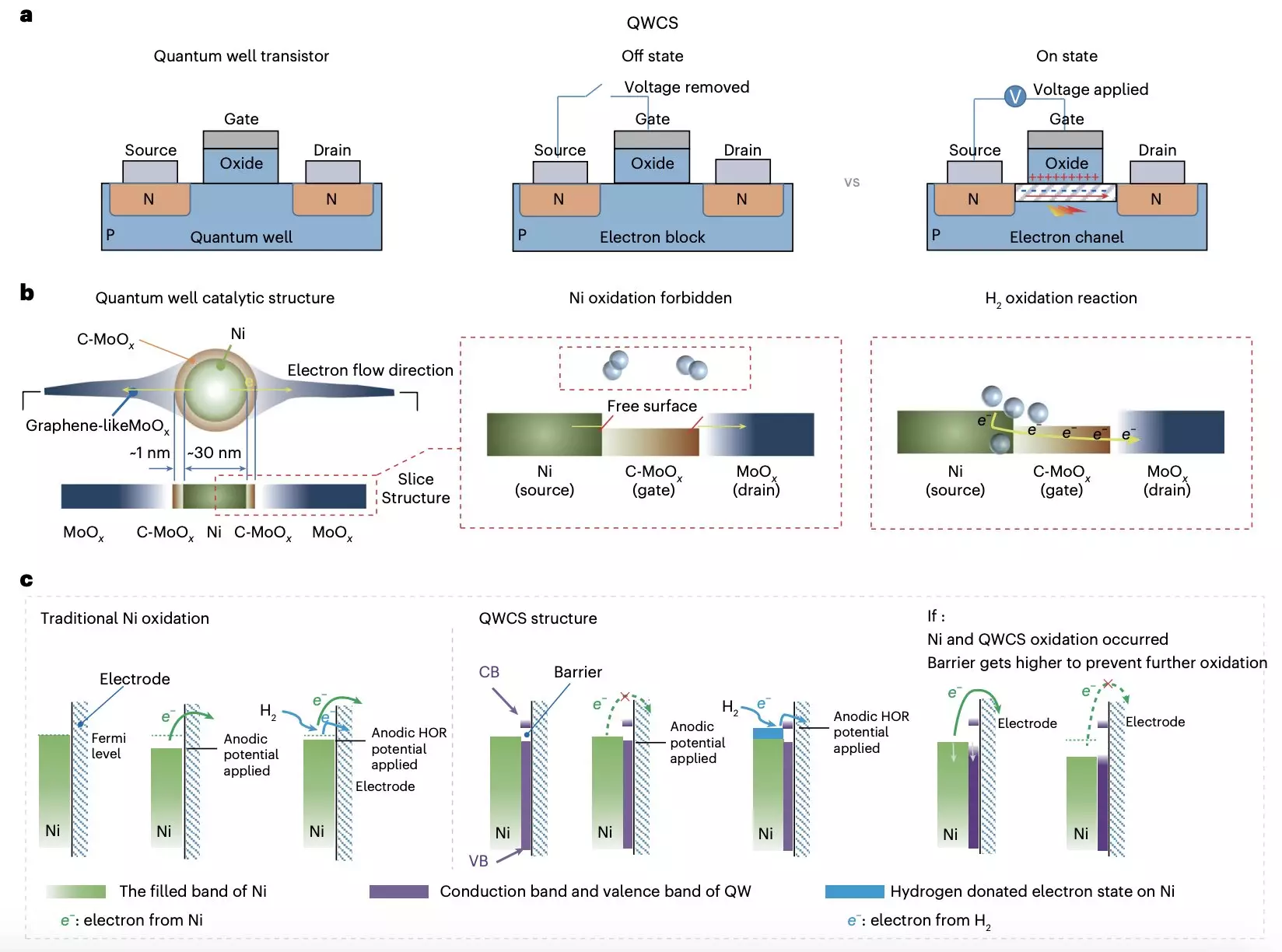
Recent breakthrough research conducted by experts at Chongqing University and Loughborough University introduces a novel strategy to combat the oxidation of metallic nickel electrocatalysts in AEMFCs. The team devised a quantum well-like catalytic structure (QWCS) that features quantum-confined metallic nickel nanoparticles, providing a solution to the self-oxidation problem. This innovative approach, outlined in a paper published in Nature Energy, showcases how the QWCS can effectively transfer external electrons from the hydrogen oxidation reaction while maintaining its metallic properties.
The Role of Ni@C-MoOx Catalyst
The heart of this research lies in the development of a new catalytic structure called Ni@C-MoOx, where nickel nanoparticles are atomically confined within a heterojunction of carbon-doped MoOx (C-MoOx) and amorphous MoOx. This unique catalyst exhibits the ability to selectively transfer external electrons generated during the hydrogen oxidation reaction without compromising the stability of the nickel catalyst itself. The robust nature of Ni@C-MoOx against electro-oxidation ensures the longevity and efficiency of fuel cells, preventing degradation and failure over time.
Promising Results and Future Implications
The Ni@C-MoOx catalyst has proven its mettle by sustaining excellent catalytic stability during continuous operation for 100 hours under challenging conditions. When utilized to create an anode-catalyzed alkaline fuel cell, the results were exceptional, with a high specific power density of 486 mW mgNi-1 and consistent performance even after repeated shutdown-start cycles. The utilization of quantum confinement in the design of this catalyst has paved the way for high-power density AEMFCs that can withstand harsh operational conditions without compromising performance.
The introduction of quantum-confined metallic nickel nanoparticles in the form of Ni@C-MoOx catalyst marks a significant leap in the realm of fuel cell technology. The innovative design strategy implemented by the research team not only addresses the issue of electro-oxidation in non-precious metal catalysts but also sets the stage for the development of cost-effective and reliable AEMFCs that hold immense promise for the future. This breakthrough is not only a testament to the power of scientific innovation but also a stepping stone towards a greener and more sustainable energy future.
Leave a Reply