The quest for renewable energy sources has led researchers to explore innovative techniques that can convert carbon dioxide (CO2) into usable fuels. Among these techniques, a revolutionary artificial photosynthesis system developed at the University of Michigan has shown significant potential by effectively binding carbon atoms together, thus transforming CO2 into ethylene. This development represents an essential step in creating sustainable fuels while concurrently addressing environmental concerns associated with carbon emissions.
At the heart of sustainable fuel production lies the ability to convert CO2, a prevalent greenhouse gas, into valuable hydrocarbons. The artificial photosynthesis system from the University of Michigan demonstrates remarkable efficiency, producing ethylene—a fundamental building block in the production of plastics. According to Professor Zetian Mi, the system’s activity and stability outshine traditional methods by five to six times, highlighting its capability to outperform existing technologies focused on CO2 reduction.
Ethylene, the most produced organic compound globally, is conventionally derived from fossil fuels through processes that are not only energy-intensive but also generate substantial CO2 emissions. By reimagining this process through artificial photosynthesis, the university’s innovation offers a promising pathway to both mitigate atmospheric CO2 and create essential industrial materials—potentially revolutionizing the plastics industry.
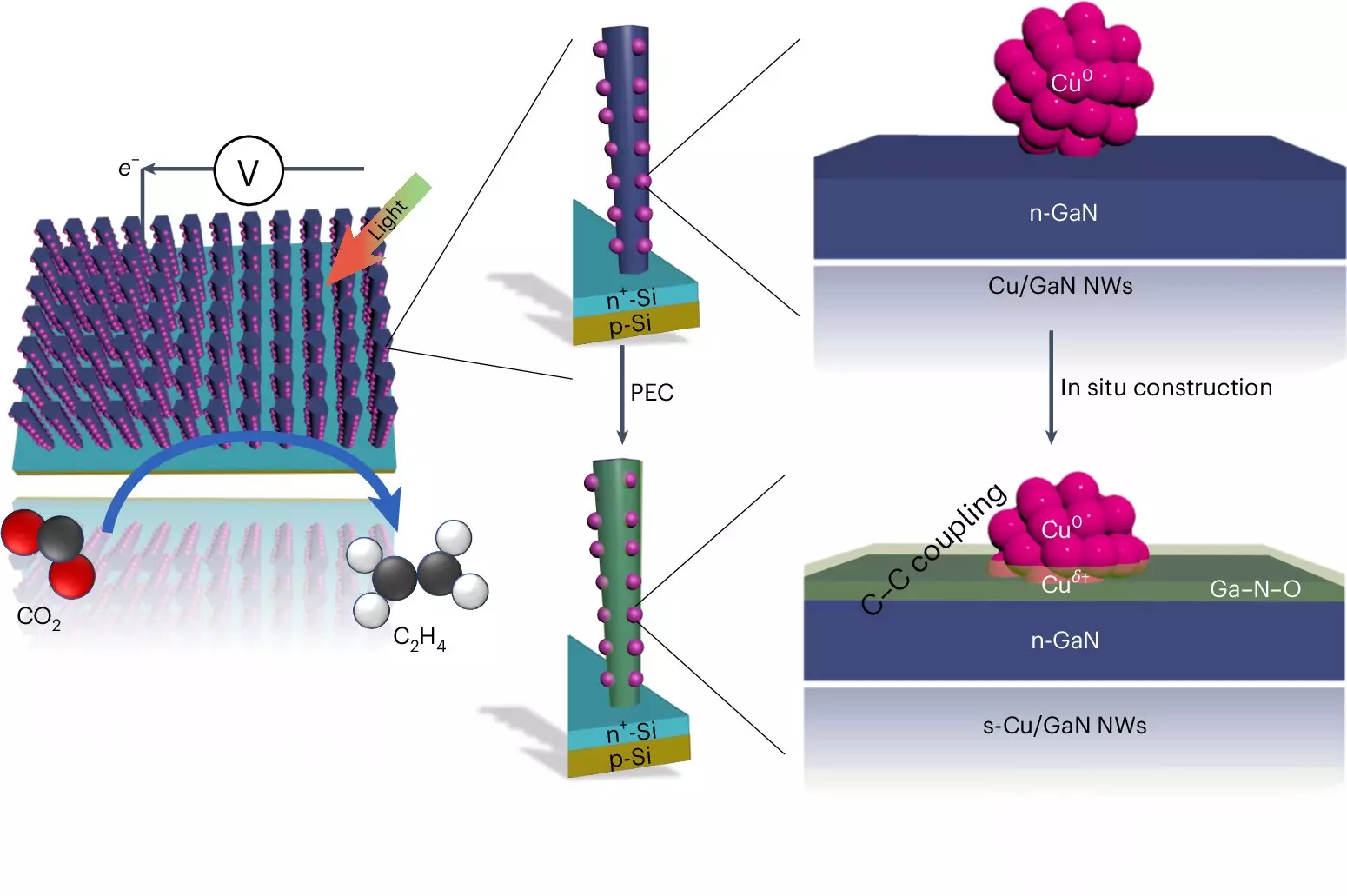
The mechanism employed by the Michigan team’s artificial photosynthesis system relies heavily on cutting-edge material technology. The device utilizes two distinct types of semiconductors: finely structured gallium nitride (GaN) nanowires and a silicon base. These components work together to facilitate a photochemical reaction that converts carbon dioxide and water into ethylene.
What sets this system apart is its efficient light absorption; the nanowires absorb sunlight and harness it to catalyze the splitting of water molecules. This process generates both hydrogen—critical to the ethylene synthesis—and oxygen, which is subsequently absorbed back into the gallium nitride, forming gallium nitride oxide. Crucially, the use of copper clusters enhances the reaction by effectively combining carbon monoxide, formed from CO2, with hydrogen, leading to the production of ethylene.
Recent findings indicate an impressive 61% of the energized electrons from the semiconductors contribute to the synthesis of ethylene, underscoring the efficiency of this innovative process. Notably, while other catalytic approaches have demonstrated similar levels of efficiency, they often face limitations in terms of reaction duration or operational requirements.
One of the most significant advantages of the University of Michigan’s system is its longevity. Traditional methods, such as those relying on silver and copper catalysts, suffer from rapid degradation and limited operational times. In contrast, the team’s apparatus sustained a continuous operation for 116 hours, with some models demonstrating functionality for up to an astonishing 3,000 hours. This durability is attributed to the synergistic relationship between gallium nitride and the water-splitting process, which fosters a self-healing mechanism that improves catalyst efficiency over time.
This ability to maintain operational integrity correlates directly with the ongoing pursuit of sustainable practices in energy production. The longer the system can operate without significant wear, the more feasible it becomes for large-scale applications, paving the way for potentially transformative impacts on the industry.
As the research team looks ahead, they are eager to explore the production of multi-carbon compounds beyond ethylene, such as propanol. The pursuit of liquid fuels represents a critical objective in the transition to sustainable energy sources. Achieving this transition could drastically alter current transportation modalities, allowing them to operate on renewable fuels, thereby reducing dependence on fossil fuels.
By effectively harnessing CO2 emissions, this cutting-edge technology illustrates the potential of artificial photosynthesis not only to create high-demand products but also to contribute to broader environmental sustainability goals. In a world grappling with climate change and increasing pollution, innovations like the one emerging from the University of Michigan could serve as vital solutions for securing a cleaner and more sustainable future.
The advancements in artificial photosynthesis herald a promising development in the journey toward sustainability. As the University of Michigan continues to refine its technologies, the opportunity arises to potentially reverse the environmental damage caused by fossil fuel consumption while simultaneously addressing ongoing material needs in industries worldwide. The findings present a beacon of hope for a more sustainable future by offering practical applications rooted in innovation.
Leave a Reply