The quest to understand nuclear stability has led scientists to investigate various isotopes, particularly those of heavy elements such as plutonium. Recently, a team from the Institute of Modern Physics (IMP) at the Chinese Academy of Sciences has made a significant breakthrough by synthesizing plutonium-227. Not only does this achievement mark a notable milestone in isotopic research, but it also offers insights into the elusive shell structure of atomic nuclei. This article delves deeper into the ramifications of this discovery and the innovative methodologies employed in the research.
The concept of shell closure in nuclear physics pertains to the stability conferred by specific configurations of protons and neutrons within an atomic nucleus. Certain “magic numbers”—including 2, 8, 20, 28, 50, 82, and 126—indicate levels where nucleon pairing leads to more stable isotopes. Historically, research has suggested a gradual weakening of neutron shell closures as one moves into the transuranium elements. This raises pivotal questions about whether similar closures persist beyond uranium and into isotopes like plutonium.
According to Prof. Gan Zaiguo from IMP, previous experiments have hinted at shell closures within neptunium isotopes, yet substantial gaps exist when it comes to plutonium’s structural integrity due to a lack of experimental data. As such, the need for further exploration into these isotopes has become pressing.
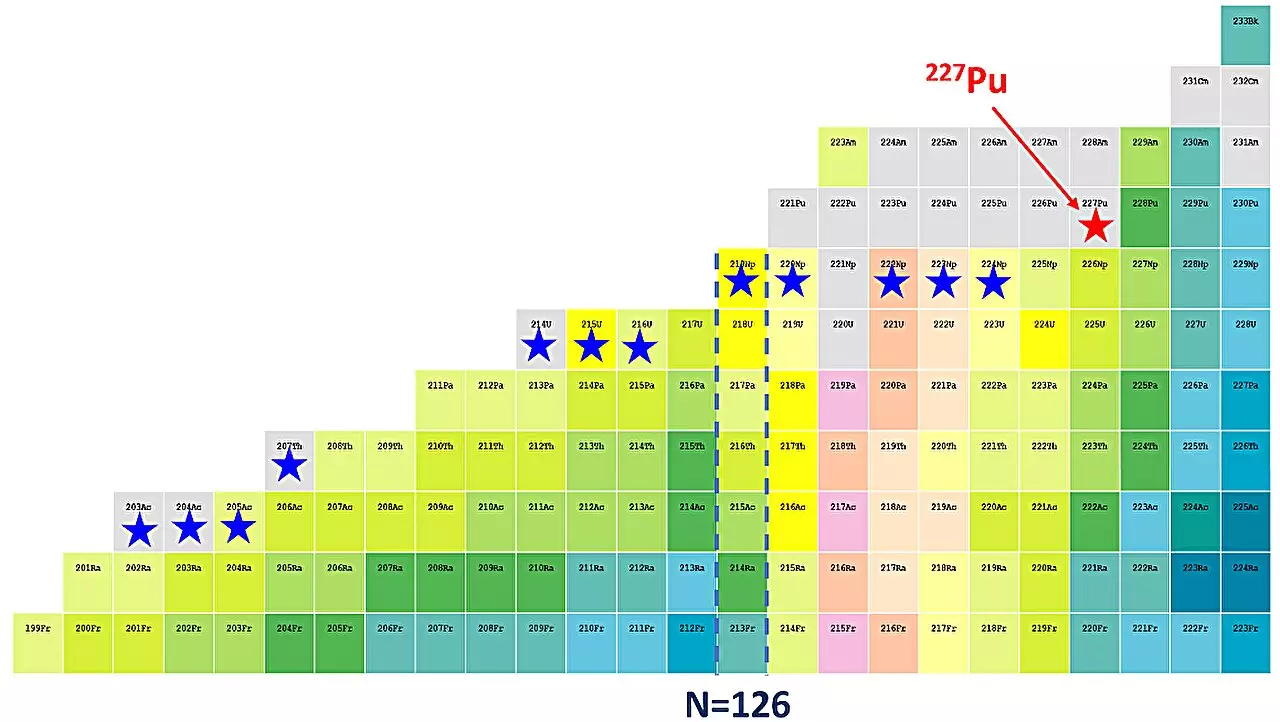
To deepen their exploration, the IMP researchers collaborated with others and conducted experiments at Lanzhou’s Heavy Ion Research Facility. Utilizing a method known as fusion evaporation, they successfully synthesized plutonium-227, which stands out as a notably neutron-deficient isotope. This achievement not only represents the first discovery of a plutonium isotope by Chinese scientists but also marks the 39th new isotope found by the IMP.
From the decay chains of plutonium-227 observed during experiments, the researchers noted the neutron emission and measured critical parameters such as the α-particle energy at approximately 8191 keV and a half-life of 0.78 seconds. These findings align well with existing data on plutonium isotopes and potentially validate the theoretical models predicting their behavior.
Profound questions and challenges remain concerning the robustness of shell closures among plutonium isotopes. Dr. Yang Huabin, the lead author of the study, emphasizes the importance of investigating lighter isotopes, specifically plutonium-221 through plutonium-226, to build a comprehensive understanding of nuclear structure in this region. The ongoing research may also offer insights that extend beyond plutonium, shedding light on the complex behavior of superheavy elements and their applications in various fields, including energy production and medicine.
The synthesis of plutonium-227 by the IMP research team highlights not just a technological achievement but also a critical step toward unlocking the mysteries of heavy element chemistry. As nuclear physicists continue to probe the complexities of isotope behavior, each discovery holds the potential for significant advancements in our understanding of atomic structure and its practical applications. Future research into isotopes like plutonium-227 could well redefine our knowledge of nuclear physics and the stability of atomic nuclei.
Leave a Reply