Transcranial focused ultrasound (tFUS) represents a groundbreaking approach in the field of neurology, providing hope for patients with conditions that have stubbornly resisted conventional treatments. By harnessing the power of high-frequency sound waves directed precisely at targeted areas within the brain, researchers hope to pave new pathways in managing neurological disorders, predominantly drug-resistant epilepsy and other tremor-related conditions. Recent contributions from a collaborative team at Sungkyunkwan University, the Institute for Basic Science, and the Korea Institute of Science and Technology bring forward an innovative sensor that could potentially transform tFUS applications.
Historically, the application of sensors on the brain has been plagued by significant limitations, particularly related to accurately capturing neural signals from the complex topology of the cortical surface. Previous devices were unable to mold adequately to the intricate folds and curvatures of the brain, resulting in unreliable measurements. Donghee Son, the supervising author of the recent study published in Nature Electronics, articulated the persistent struggle of earlier sensor designs: while a previous sensor developed by Professors John A. Rogers and Dae-Hyeong Kim offered some improvements in signal accuracy, it still couldn’t conform tightly enough to highly curved regions of the brain. Such shortcomings have made the rigorous diagnosis of brain lesions and the continuous monitoring of neural activity exceedingly difficult.
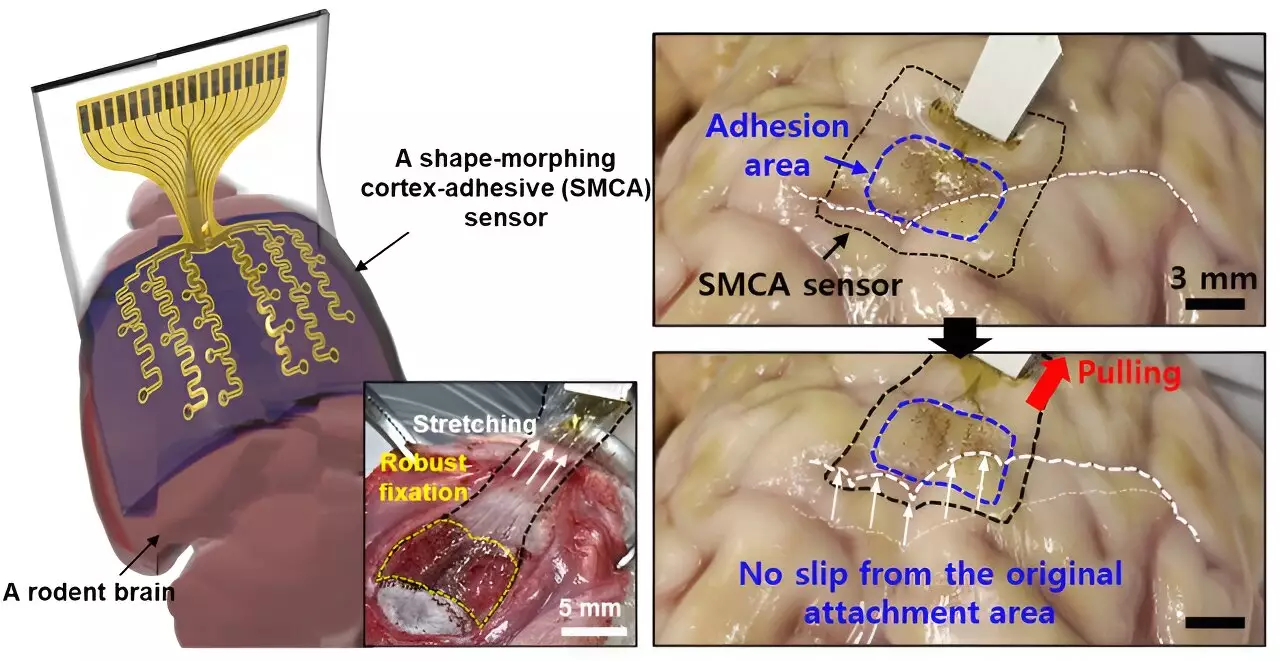
Moreover, earlier sensor systems were susceptible to movement. Micro-motions of the brain combined with fluctuations in cerebral spinal fluid could compromise their attachment, leading to inaccurate readings. The quest, therefore, for a more effective sensor that could maintain stable adhesion across varying brain surface complexities has remained elusive—until now.
Addressing these significant barriers, Son and his fellow researchers developed a cutting-edge sensor, dubbed ECoG, that possesses the ability to mold itself to the unique curves of the brain. This feature directly correlates to improved measurement reliability and longevity of readings, which are crucial for effective treatment, particularly in the management of epilepsy. As stated by Son, “The new sensor we developed can tightly conform to highly curved brain regions and adhere firmly to the brain tissue,” thereby facilitating real-time data collection and targeted stimulation.
The ECoG sensor comprises three innovative layers: a hydrogel-based layer that creates chemical and mechanical bonds with brain tissue, a self-healing polymer layer that adapts its shape to follow the brain’s curvature, and a stretchable layer with gold electrodes for signal monitoring. This multifaceted approach allows the device to maintain a void-free contact with the brain surface, significantly reducing noise that could disrupt the efficacy of tFUS applications.
One of the most compelling aspects of this advancement lies in its implications for personalized medicine. In recent years, there has been a notable push towards tailoring treatment approaches to match individual patient profiles, particularly concerning neurological disorders. Achieving this objective emphasises the necessity for real-time monitoring capabilities that can accurately assess the brain’s activity while also applying stimulation.
Son highlighted the challenges faced by conventional sensors, stating, “Conventional brain surface-attached sensors struggled with this because the ultrasound-induced vibrations caused significant noise.” The introduction of the new sensor significantly minimizes this noise, facilitating more tailored and effective treatment options.
Initial tests on awake rodent subjects yielded promising results, showcasing the sensor’s capability to monitor brain waves and regulate seizure activity efficiently. This early success not only positions the ECoG sensor as a valuable tool in the treatment of epilepsy but also opens the door for future applications in diagnosing various other neurological conditions.
Looking ahead, the researchers aim to enhance the sensor’s specifications by creating a high-density electrode array. This aims to amplify the device’s ability to map brain signals with greater resolution, catering to increasingly complex diagnostic and therapeutic needs. Son envisions broad applications for this technology, stating, “We expect this technology will not only be applicable in epilepsy treatment but also in diagnosing and treating various brain disorders.”
The advent of the ECoG sensor and its innovative design features promise to reshape the landscape of neurological treatments significantly. Not only does it aim to offer immediate interventions for conditions such as epilepsy, but its adaptable nature also heralds a broader spectrum of therapeutic possibilities. The concerted efforts evidenced by the collaborative research team signal a hopeful future, where individualized patient care might just become a reality in the realm of neurological healthcare. As this research progresses through clinical trials, it could revolutionize how we approach treatment, diagnosis, and ultimately, patient outcomes in the field of neurology.
Leave a Reply