At the heart of both natural processes and human-made technologies lies a phenomenon that is both fascinating and essential: the conversion of energy through charge transfer. In nature, photosynthesis equips plants and certain bacteria with the remarkable ability to harness sunlight, facilitating their growth and sustenance. Meanwhile, solar panels employ photovoltaic technologies to directly transform light into electrical energy, providing a clean source of power. Both processes hinge on electronic movement, which fundamentally involves the transfer of charge at the molecular level. Understanding the mechanisms that drive these processes on an ultrafast timescale—ranging from femtoseconds to attoseconds—has profound implications for multiple fields, including quantum chemistry and materials science.
The peculiar dance of electrons within molecules is governed by the principles of quantum mechanics, and it plays a crucial role in defining how energy is absorbed and utilized in chemical reactions. Notably, when molecules absorb photons, the electronic density within them redistributes almost instantaneously. This ultrafast dynamic phenomenon, while conceptually understood, has remained challenging to observe and analyze due to its brevity and complexity. Cutting-edge imaging techniques with extreme temporal resolution are now being developed to capture these fleeting events. The innovative use of ultrashort ultraviolet pulses from advanced high-order harmonic sources or free electron laser facilities offers a means to initiate and scrutinize molecular responses to photonic stimuli over an impressively brief time interval, spanning from 10^-15 to 10^-18 seconds.
However, as much progress as has been made, a comprehensive understanding of the initial phases of electron and charge transfer following photoionization continues to be elusive. Newly published research from the Politecnico di Milano, alongside several esteemed institutions in Madrid, aims to bridge this knowledge gap.
Breakthroughs in Attosecond Science
The study, as reported in Nature Chemistry, showcases pioneering methods of observing electron dynamics using attosecond extreme-ultraviolet (XUV) pulses. This research provides a novel perspective on the interaction between nuclei and electrons in donor-acceptor molecular systems, pushing the boundaries of our comprehension of fundamental chemical processes. By targeting nitroaniline molecules with these attosecond pulses, the research team successfully visualizes and evaluates the initial moments of charge transfer, revealing unparalleled intricacies in the electron-nuclear coupling dynamics.
Utilizing a sophisticated combination of techniques, such as attosecond XUV-pump/few-femtosecond infrared-probe spectroscopy and advanced many-body quantum chemistry, the researchers chart electron movements with remarkable precision. These methodologies not only track electron transfers but also elucidate the temporal phases of shifting electronic states within molecules, which until now have remained obscured in scientific literature.
Key Discoveries in Charge Transfer Dynamics
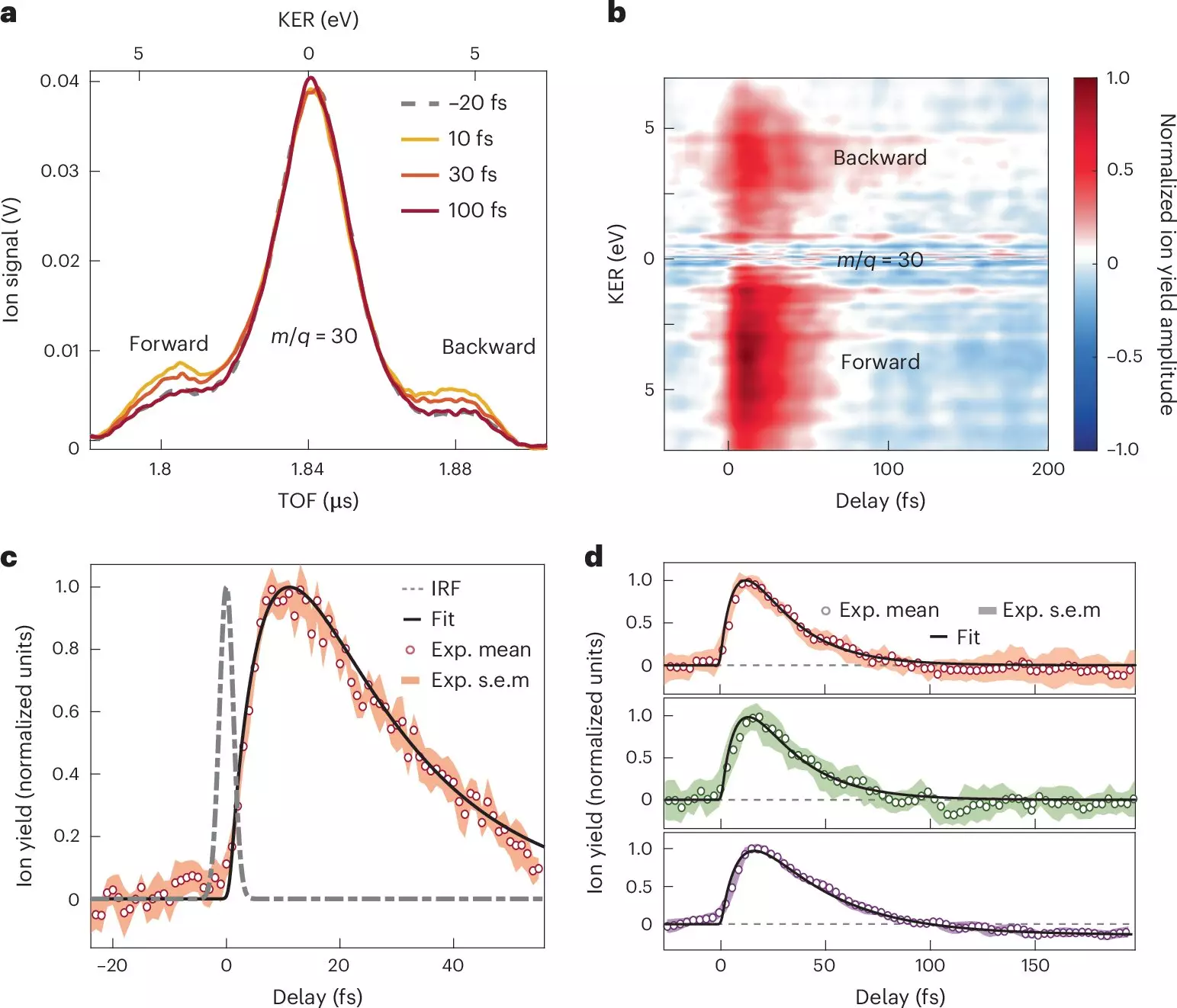
The results from this investigation identify crucial timeframes in the charge transfer process. Specifically, it was found that electron transfer from the electron donor amino group transpires in less than 10 femtoseconds, underscored by a synchronized orchestration of nuclear and electronic motion. Following this rapid transfer, a relaxation phase emerges within a sub-30-femtosecond interval, characterized by the nuclear wave packet’s dispersion across the excited states of the molecular cation.
These findings are monumental as they reveal the mechanics behind charge migration in complex molecular architectures, providing new insights into how structural rearrangements accompany electron transfer. This understanding challenges traditional models that often oversimplify interactions in organic molecules and lays a foundation for revising this textbook knowledge to reflect more nuanced behaviors.
The implications of the study extend beyond fundamental research to practical applications in attosecond science and the development of materials with tailored electronic properties. By elucidating the dynamics of charge transfer, future innovations could optimize organic photovoltaics, enhance catalysis, and refine strategies for energy storage. The delicate relationship between electrons and nuclei observed in this study could inspire the design of novel materials capable of efficient energy conversion processes.
This groundbreaking research not only advances our comprehension of molecular dynamics at unprecedented timescales but symbolizes a step forward in our quest to harness the intricate dance of electrons for energy conversion and technology development. As we continue to explore the quantum realm, the potential for transformative discoveries remains boundless.
Leave a Reply