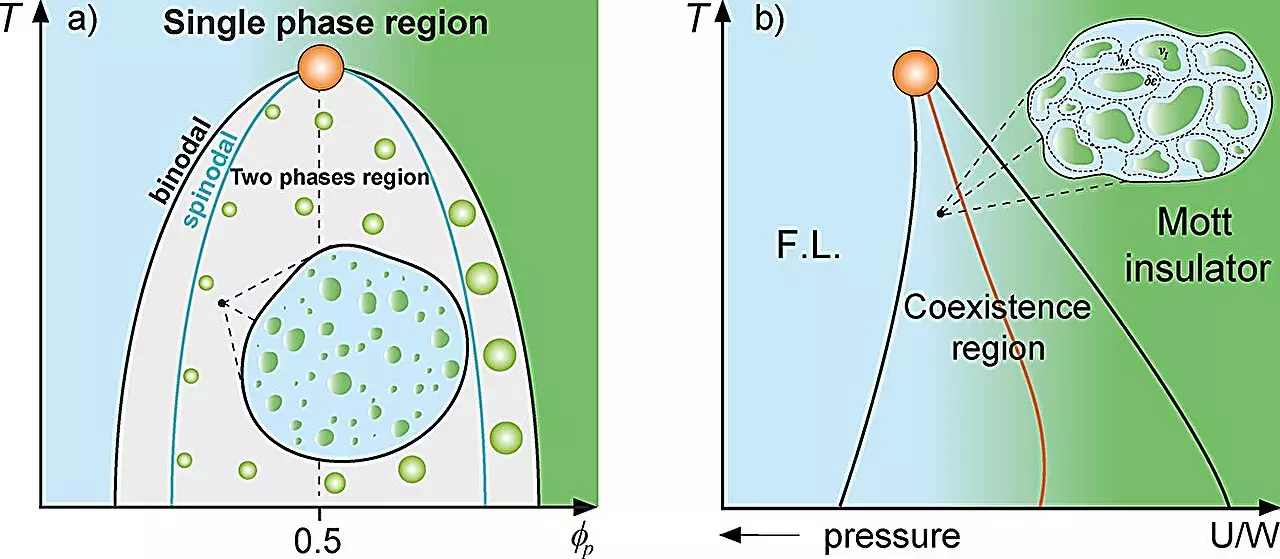
In the realm of physics, classical mixture theory serves as a foundational framework for analyzing systems composed of different substances. This theory is particularly relevant when investigating how distinct materials interact and coexist, especially under varying conditions. For example, consider the behavior of water when subjected to supercooling: it can exhibit phases of high and low density, showcasing the complex interplay between liquid states. Similarly, the Mott metal-insulator transition illustrates how metallic droplets can emerge within an insulating medium. Such phenomena highlight the fundamental principles governing the interactions and distribution of components within a mixture, shedding light on both physical and biological systems.
A recent study conducted by researchers at São Paulo State University (UNESP) in Brazil brings these concepts into the biological sphere, specifically in the context of protein compartmentalization within cells. By drawing parallels between the Griffiths phase observed in magnetism and the behavior of proteins in cellular environments, the researchers propose a novel framework for understanding how proteins behave in various cellular contexts. Under the leadership of Mariano de Souza, a distinguished professor at UNESP, and Ph.D. candidate Lucas Squillante, this research sheds light on how proteins can form distinct compartments or droplets within cells, akin to “rare regions” found in physical mixtures.
As proteins accumulate within a cell, they can reach a threshold where they undergo liquid-liquid phase separation, leading to the formation of these protein droplets. This process is crucial, as it influences how proteins interact with one another and their respective functions. The researchers employed thermodynamic models such as the Grüneisen parameter and the Flory-Huggins model to analyze these complex dynamics further, revealing that protein compartmentalization significantly reduces the stochastic dynamics of cellular processes.
In an intriguing proposed extension of their findings, the study posits a link between the Griffiths-like cellular phase and the origins of life itself. Referencing the pioneering work of Russian biologist Aleksandr Oparin, the researchers suggest that primordial life forms likely emerged within coacervates—droplets formed from organic molecules clustering in water. These nascent cellular structures with slow dynamics would have provided a unique evolutionary advantage, allowing for more stable environments conducive to the emergence of life.
The significance of chirality in this evolutionary context cannot be overstated. Chirality refers to the property of certain molecules that prevents them from being superimposed on their mirror images. In biological systems, the predominance of a single chirality (homochirality) is essential for the proper functioning of many biochemical processes. The study hints that the dynamics of protein diffusion and the resulting cellular behavior shaped by these chiral molecules may have influenced the paths taken by early life on Earth.
The implications of this research extend far beyond theoretical musings about the origins of life; they have significant ramifications for understanding contemporary health issues. The authors emphasize that liquid-liquid phase separation plays a vital role in a myriad of diseases, including cancer and neurodegenerative disorders. Protein compartmentalization within cells can alter the behavior of proteins linked to these diseases, ultimately impacting cellular mutation and disease progression.
For example, the study highlights that phase separation phenomena are linked to various health-related conditions, including cataracts, where phase separation in the lens of the eye leads to visual impairment, and even COVID-19, where irregularities in protein droplet formation can hinder immune responses. These insights demonstrate how understanding the underlying physics of cellular dynamics can inform potential therapeutic interventions and strategies for disease management.
This groundbreaking work, which encompasses input from multiple disciplines, illustrates the power of interdisciplinary research in unraveling complex biological phenomena. The collaboration among researchers from different universities and backgrounds underscores the importance of collective expertise in advancing our understanding of cellular processes.
As further exploration in this field progresses, the potential for applications in disease treatment and management becomes increasingly apparent. The Griffiths-like cellular phase concept introduced by Souza and his colleagues may lead to innovative strategies aimed at harnessing the principles of phase separation for therapeutic purposes. The promise of such an approach serves as a reminder that even the most complex biological systems can be analyzed through the lens of fundamental scientific principles.
The exploration of protein compartmentalization as influenced by Griffiths-like phases offers a rich avenue for understanding both life’s origins and the intricacies of health and disease. This study not only bridges physics and biology but also sets the stage for future innovations that could revolutionize our approach to many pressing biomedical challenges.
Leave a Reply