Radiation has a profound impact on water, and it is a topic that has intrigued scientists for a considerable time. Recently, a team of theoretical physicists at DESY, German Electron Synchrotron, sought to uncover the mysteries behind the interaction of radiation and water. By analyzing data collected by their colleagues at Argonne National Laboratory in the United States, the team made groundbreaking discoveries that shed light on the behavior of free electrons in water at short time scales. These findings have significant implications in the field of physics and have been published in the Journal of the American Chemical Society.
One of the key questions addressed by this research is the behavior of free electrons in water. Free electrons are electrons that are not bound to atoms and are released when water molecules come into contact with radiation. The movement of these electrons between water molecules has been a subject of debate and speculation among physicists. The experimental team, led by Argonne scientist Linda Young, conducted experiments at the LCLS X-ray laser in California to observe the behavior of water molecules under the influence of lasers and X-ray radiation. Their observations revealed peculiar signatures and structures within the water molecules, which sparked the need for further examination.
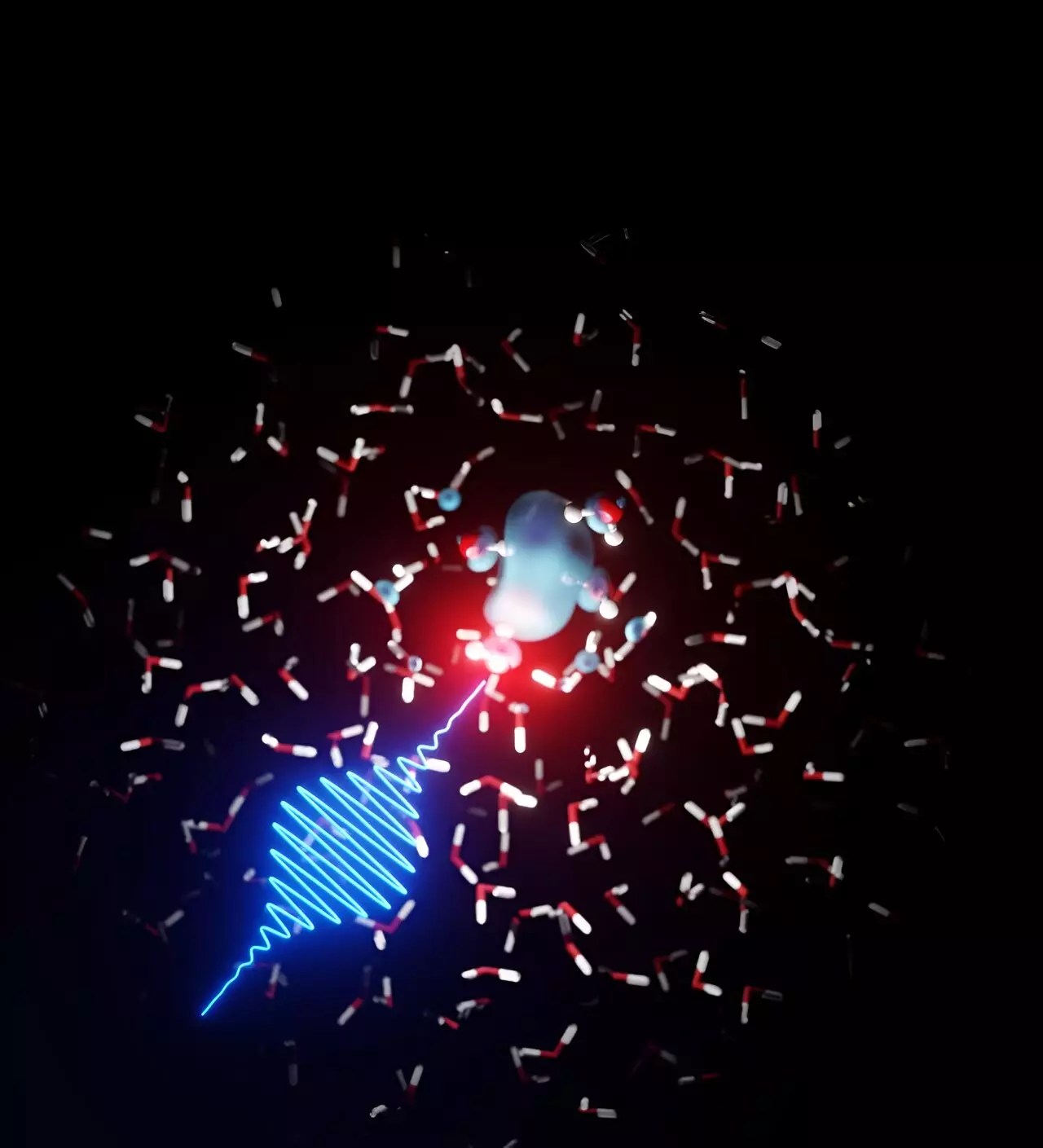
To gain a deeper understanding of the observed phenomena, the experimental team collaborated with theoretical physicists from DESY. Led by scientist Ludger Inhester from the Center for Free-Electron Laser Science, the theoretical team thoroughly analyzed the data and developed models to explain the observed structures. Through their collaboration, the teams discovered that the free electrons in water form bubble-like structures, which are subsequently enveloped by neighboring water molecules, akin to the solvation of chemicals in water at the molecular level. Using X-ray absorption spectroscopy, the DESY team successfully elucidated the process and parameters governing the solvation of electrons in water.
One intriguing finding from the study is the remarkable sensitivity of the dissolution process and the formation of cage-like structures to changes in water temperature. The dissolved electrons, initially dispersed among the water molecules, eventually anchor themselves to specific hydrogen-bonding patterns within the liquid water. The localization of the electrons prompts a rapid reorientation of neighboring water molecules, a process completed within an astonishingly short timescale of 100 femtoseconds. It is worth noting that a femtosecond is equivalent to a quadrillionth of a second. The bubble-like structures, measuring approximately 50 billionths of a meter, disintegrate within a few picoseconds, or a trillionth of a second.
Understanding how water reacts when exposed to radiation is crucial, as it forms the basis for subsequent radiation chemistry, a phenomenon that is also applicable to biological materials. The new findings offer valuable insights into the initial steps of chemical reactions driven by radiation in water. This knowledge contributes to a deeper understanding of radiation damage caused by ionizing radiation in water, a topic of great relevance in various scientific disciplines. Consequently, there are plans to deepen water-related research at the emerging Center for Molecular Water Science, a collaborative effort on the DESY campus.
The collaborative efforts of the teams from DESY and Argonne National Laboratory have uncovered the secrets of radiation and water interaction. Their research provides new perspectives on the behavior of free electrons in water and reveals fascinating details about the solvation process and its dependence on temperature. Moreover, the findings have implications for understanding radiation damage in water and its effects on biological materials. As these discoveries pave the way for further exploration, the establishment of the Center for Molecular Water Science signifies the growing importance of water-related research in scientific endeavors.
Leave a Reply